Gallego M, Botella LM, Mascaraque M, Nicolás J, Albiñana V, Abarca E, Gilaberte Y, González S, Juarranz Á, Carrasco E. N-acetylcysteine and raloxifene boost photodynamic therapy against cutaneous squamous cell carcinoma by decreasing TGFβ1 secreted by cancer-associated fibroblasts
Int J Biol Sci. 2025
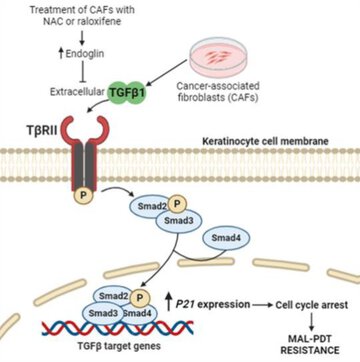
"TGFβ1 levels in the tumor microenvironment emerge as a crucial indicator to anticipate the responsiveness of squamous cell carcinoma to PDT, posing NAC and raloxifene as promising candidates to overcome therapeutic resistance by inducing endoglin expression in cancer associated fibroblasts. - Dr Elisa Carrasco
Summary:
Cutaneous squamous cell carcinoma (cSCC) is a highly prevalent skin cancer. While surgery remains the gold standard treatment, non-invasive methods like photodynamic therapy (PDT) stand out for their high efficacy and minimal cosmetic impact. However, resistance to PDT is still a challenge. Numerous cellular processes involved in cancer biology and therapy resistance are regulated by the TGFβ1/SMAD pathway. Using in vitro bidimensional and tridimensional cultures of cSCC cell lines, we studied the development of resistance to PDT in response to TGFβ1 secreted by cancer associated fibroblasts. Our results highlight the TGFβ1 co-receptor endoglin as a key molecular player in the process. Importantly, targeting endoglin expression with N-acetylcysteine (NAC) or raloxifene significantly reduced TGFβ1 levels and effectively prevented resistance. In addition, the combination of PDT with NAC resulted in an improved therapeutic outcome in vivo in SKH-1 mice with cSCC photogenerated by chronic exposition to ultraviolet light. In conclusion, the combination of PDT with NAC or raloxifene enhances PDT efficacy by mitigating resistance mechanisms, which can open new avenues for the treatment of cSCC.
Why do you highlight this publication?
We unveil the co-adjuvant potential of N-acetylcysteine and raloxifene to enhance the efficacy of photodynamic therapy in the treatment of squamous cell carcinoma. By using in vitro and in vivo models, our work underscores the ability of these drugs to induce an endoglin-mediated reduction of the extracellular TGFβ1 levels derived from cancer associated fibroblasts.
Publication commented by:
Dr Elisa Carrasco Cerro
Autonomous University of Madrid
EXPERIMENTAL DERMATOLOGY AND CUTANEOUS BIOLOGY Group-IRYCIS