Mario Romero-Rivera, Miguel D. Fernández-de-Bobadilla, María Beltrán, Rosa del Campo, José Avendaño-Ortiz and Cristina Herencias. Genome assembly and functional predation analysis of novel Bdellovibrio isolates from human gut microbiota
Front. Microbiol. 2026
"Human‑derived predatory bacteria pave the way to 'living antibiotics' that can attack multidrug‑resistant pathogens while preserving the gut microbiota". - Mario Romero-Rivera and Dr. Cristina Herencias Rodríguez
Summary:
Introduction: Predatory bacteria of the Bdellovibrio and like organisms (BALOs) have long been postulated as living antimicrobials, yet their occurrence and ecological roles within human-associated microbiota have remained uncertain due to the absence of culturable human-derived isolates. Here, we report the first successful isolation and comprehensive characterization of viable Bdellovibrio bacteriovorus from human fecal samples.
Methods: Targeted enrichment was applied to five pooled fecal samples to facilitate predator recovery. We performed whole-genome sequencing on the isolates and conducted comparative genomics across 162 publicly available Bdellovibrio genomes. Additionally, pangenome analysis of 22 high-quality genomes and phenotypic assays against clinical pathogens were conducted to assess genomic diversity, prey specificity, and biosafety profiles.
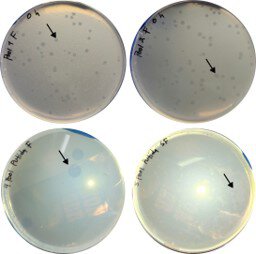
Results: Despite extremely low natural abundance, targeted enrichment recovered predators in two of five pooled samples, which produced characteristic lytic plaques. Sequencing revealed >99% average nucleotide identity to reference strain HD100 with only 26 core single-nucleotide polymorphisms across both isolates, indicating minimal divergence between human-associated and environmental lineages. Comparative genomics showed that only 10.4% of public genomes fulfill criteria for B. bacteriovorus sensu stricto. Pangenome analysis revealed a stable, highly conserved core (~2,500-2,650 genes) and an expanding accessory genome. Phenotypically, the human-derived isolates displayed narrower prey ranges concentrated on Pseudomonas spp., including multidrug-resistant clinical strains, and no acquired virulence factors were detected.
Discussion: Collectively, these findings suggest predation in the human gut and that viable Bdellovibrio could be natural, genomically conserved members of the intestinal ecosystem. This work advances a testable keystone-predator framework for human microbiome ecology and opens an ecologically informed therapeutic pathway in which human-associated Bdellovibrio may help control multidrug-resistant pathogens while supporting microbiota homeostasis.
Why do you highlight this publication?
This study reports the first successful isolation and in‑depth genomic and functional characterization of viable Bdellovibrio bacteriovorus from the human gut, closing a long‑standing gap between molecular detection and true cultivation of these predatory bacteria. The human‑derived isolates are almost identical at the genomic level to the classical HD100 reference strain and do not harbour acquired virulence determinants, supporting a favorable biosafety profile. Functionally, they preferentially prey on Pseudomonas spp., including multidrug‑resistant clinical isolates, highlighting their potential as "living antibiotics" that can selectively target problematic pathogens. Overall, this work introduces a keystone‑predator framework for understanding human microbiome ecology and opens an ecologically informed therapeutic avenue to control Gram‑negative infections while helping preserve intestinal homeostasis.
Publication commented by:
Mario Romero-Rivera & Dr. Cristina Herencias Rodríguez
BIOLOGY AND EVOLUTION OF MICROORGANISMS. IRYCIS