González S, Palacios J, Carretero I, Fernández V, Cortés A, Román J, Matias X, Gatius S, Cortés J, Pérez B. Single-nucleus RNA sequencing identifies a novel tenogenic heterologous differentiation in endometrial carcinosarcomas: implications for diagnosis and tumor classification
J Pathol. 2026
"Single-nucleus RNA sequencing uncovers unexpected tenogenic differentiation and extreme cellular plasticity in carcinosarcomas". - Dr. Silvia González Martínez & Dr. Belén Pérez Mies
Summary:
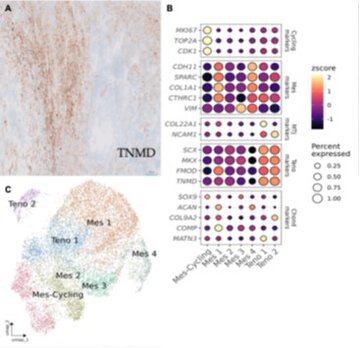
Carcinosarcomas (CSs) are aggressive biphasic tumors characterized by epithelial and mesenchymal components, whose histogenesis and differentiation dynamics remain poorly understood. We present single-nucleus RNA sequencing (snRNA-seq) analysis of six CSs (five endometrial and one ovarian) and two normal endometrial samples, profiling over 96,298 cells. By integrating transcriptomic data with inferred copy number variations (CNVs), immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and in situ hybridization (ISH) validation, we resolved the complex cellular architecture of these tumors, identified lineage-specific programs, and revealed unexpected differentiation trajectories. snRNA-seq was used to further refine the histopathological classification of three cases by uncovering heterologous differentiation not previously recognized: one rhabdomyogenic, one osteogenic, and, notably, one exhibiting a novel tenogenic program, defined by the expression of SCX, MKX, and TNMD. All CSs displayed a prominent mesenchymal compartment comprising both undifferentiated fibroblast-like cells and distinct lineage committed populations, including rhabdomyoblasts (Rhab), tenoblasts (Teno), osteoblasts (Osteo), and chondroblasts (Chond). In some tumors, multiple mesenchymal identities co-existed, and in others, differentiation gradients (e.g. immature versus mature rhabdomyoblasts) were observed. These patterns underscore the cellular plasticity and multilineage potential of the sarcomatous component. Furthermore, the expression of specialized interface markers (COL22A1, NCAM1, ACAN, CHRNG, MUSK) suggests that some tumors use structured developmental programs reminiscent of the muscle-tendon junction, enthesis, or neuromuscular junction. CNV analysis revealed tumor-specific genomic alterations with clonal and subclonal patterns linked to differentiation state, which were validated by FISH. Altogether, this study demonstrates that CSs are not static biphasic tumors but rather complex ecosystems with extensive developmental plasticity. Our findings redefine their classification and support the use of single-nucleus approaches to uncover hidden differentiation trajectories in highly heterogeneous cancers, including the discovery of a previously unreported tenogenic lineage. Our results challenge the diagnosis of homologous CS when only morphological criteria are applied. © 2026 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Why do you highlight this publication?
I highlight this publication because it represents a key conceptual and technical advance in the understanding of carcinosarcomas. By applying single-nucleus RNA sequencing to FFPE samples, this work overcomes major technical limitations and reveals an unexpected level of cellular plasticity and multilineage differentiation that cannot be captured by conventional histopathology alone.
Importantly, the study identifies a previously unrecognized tenogenic differentiation program, alongside other heterologous mesenchymal lineages, challenging the traditional classification of carcinosarcomas as "homologous" or "heterologous." These findings have direct implications for tumor classification, diagnosis, and our biological understanding of these aggressive neoplasms.
For me as an author, this publication is particularly significant because it integrates cutting-edge single-cell technologies with clinically relevant pathology questions, demonstrating how transcriptomic approaches can refine and transform diagnostic concepts in cancer.
Publication commented by:
Dr. Silvia González Martínez & Dr. Belén Pérez Mies
MOLECULAR PATHOLOGY OF CANCER GROUP. IRYCIS