S. Vano-Galvan, R. Pirmez, A. Hermosa-Gelbard, O.M. Moreno-Arrones, D. Saceda-Corralo, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients
J Am Acad Dermatol. 2021
"This multicenter study showed that low-dose oral minoxidil has a good safety profile as a treatment for hair loss. Only 1.7% of patients required discontinuation of the drug due to adverse effects". - Dr. Sergio Vañó Galván -
Summary:
Background: The major concern regarding the use of low-dose oral minoxidil (LDOM) in the treatment of hair loss is the potential risk of systemic adverse effects.
Objective: To describe the safety of LDOM for the treatment of hair loss in a large cohort of patients.
Methods: Retrospective multicenter study of patients treated with LDOM for at least 3 months as a treatment for any type of alopecia.
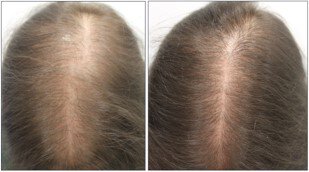
Results: A total of 1404 patients [943 women (67.2%) and 461 men (32.8%)] with a mean age of 43 years (range 8-86) were included. From them, the dose of LDOM was titrated in 1065 patients, allowing the analysis of 2469 different cases. The most frequent adverse effect was hypertrichosis (15.1%) which led to treatment withdrawal in 14 patients (0.5%). Systemic adverse effects included lightheadedness (1.7%), fluid retention (1.3%), tachycardia (0.9%), headache (0.4%), periorbital edema (0.3%) and insomnia (0.2%), leading to drug discontinuation in 29 patients (1.2%). No life-threatening adverse effects were observed.
Limitations: Retrospective design, lack of a control group.
Conclusion: LDOM has a good safety profile as a treatment for hair loss. Systemic adverse effects were infrequent and only 1.7% of patients stopped the treatment due to adverse effects.
Why do you highlight this publication?
This publication represents the largest study worldwide demonstrating the safety of low-dose oral minoxidil in different types of alopecia. Low-dose oral minoxidil is one of the most widely used emerging therapies for the treatment of alopecia nowadays. This multicenter study analyzed 1404 patients treated with this drug in six different countries. Interestingly, the risk of systemic adverse effects was low and the rate of discontinuation of the drug due to adverse effects was less than 2%.
Publication commented by:
Dr. Sergio Vañó Galván
Dermatology Service of Hospital Universitario Ramón y Cajal