- Pharmaceutical quality system subject to favorable inspection by the Spanish Agency for Medicines and Health Products (AEMPS).
- MITECO authorization for the handling of genetically modified organisms: CAR-T cells
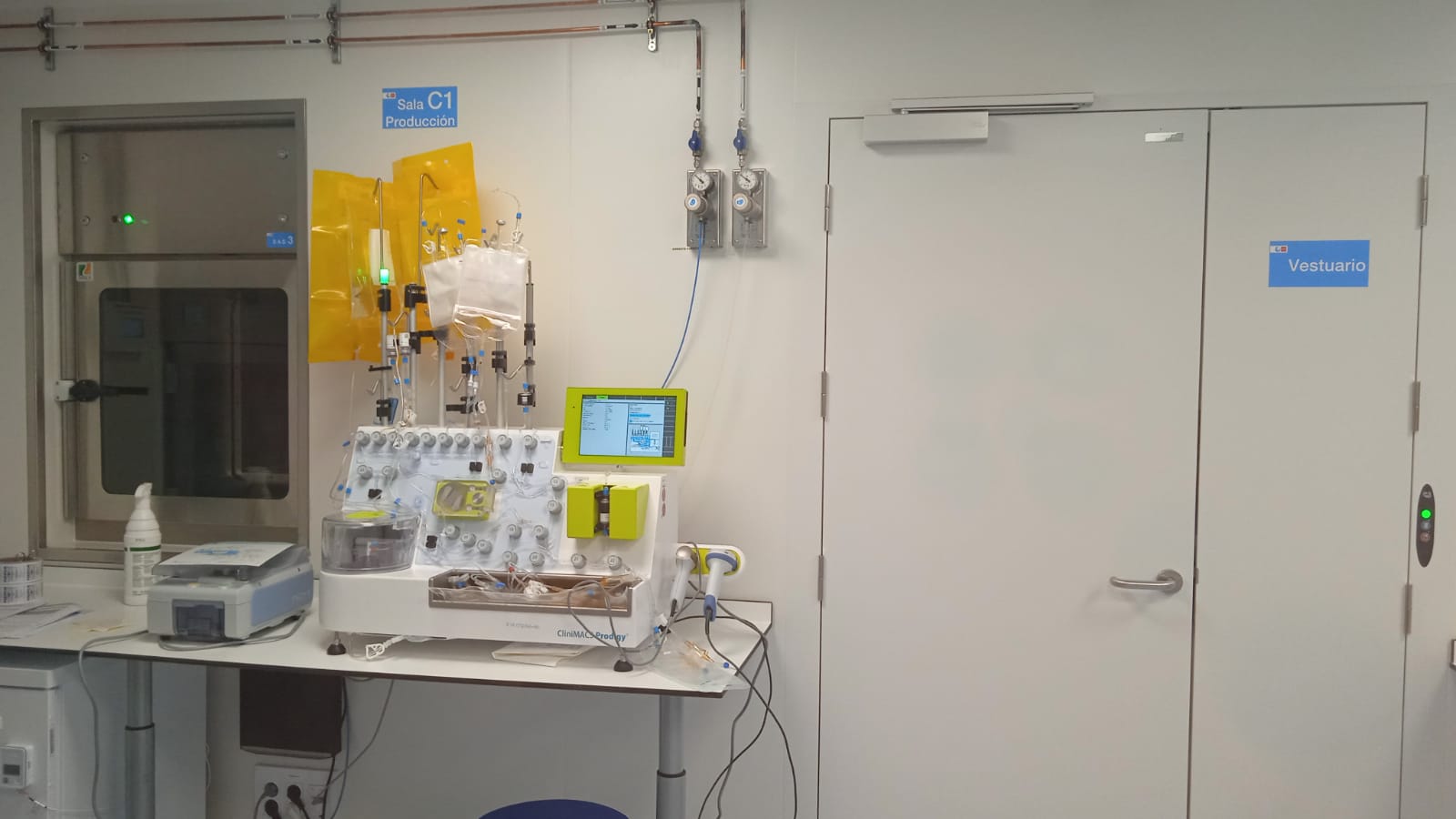
Our Cell Therapy Production Unit operates under a rigorous pharmaceutical quality system, duly certified and subject to favorable inspection by the Spanish Agency for Medicines and Health Products (AEMPS). We have state-of-the-art facilities and equipment specially designed for the aseptic production of cutting-edge cell and gene therapies-including CAR-T therapies, TILs, specific T lymphocytes, regulatory T lymphocytes, NK cells, and mesenchymal cells-with scalability, traceability, and control adapted to the highest standards. Our highly experienced team has carried out all the validation phases required by the AEMPS for the authorization of clinical trials with CAR-T therapies and specific T lymphocytes. Thanks to this comprehensive approach, our Unit is perfectly equipped to accompany the development of advanced therapies from design to clinical implementation, ensuring quality, safety, and regulatory compliance at every stage.