Biobank and biomodels

Leader
Sonia Camaño Páez
Personnel
- BIOBANCO
- Bárbara Luna (Responsable Lab.)
- BIOMODELOS
- Bruno Sainz (Coord. modelo in vivo- PDX)
- Laura Ruiz (Resp. Lab. modelo in vivo)
- Julie Earl (Coord. modelo in vitro / líneas celulares /organoides)
Contact
biobanco(ELIMINAR)@salud.madrid.org
Telf 1: 91 336 80 00 Extensión: 7955
Telf 2: 91 336 90 75
Planta -4 derecha
-
What is a biobank?
Biobanks are research support units that act as a link between donors, clinical and research staff, whose purpose is to create collections of samples and associated clinical data of different pathologies. These collections are managed by Biobank, which guarantees compliance with the established social, ethical and quality requirements. They also have the capacity to adapt to the needs of the research staff in order to be able to carry out a specific research project.
The Biobank's activity is characterised by the following:
- Biobank authorised by the Regional Ministry of Health (National Biobank Register B.0000678).
- Process control: Quality Management System according to the UNE-EN ISO 9001:2015 standard. Certified by SGS ICS (No. ES13/14407).
- To guarantee the quality, security and traceability of the data and biological samples stored.
- Compliance with the applicable legislation and regulations, to guarantee the rights of donors.
- Computer management with high-level security conditions. Integral management of processes, traceability of samples and associated data.
- Registration of the pre-analytical conditions of the samples (International SPREC code and conditions of the samples).
- International coding of pathologies
- Standardised working protocols
- Control and security of isothermal media, connected to a generator, with continuous temperature measurement and recording, alarm management and contingency procedures.
- Coordination capacity between different services/units for the generation of complex collections.
- Establish connections between research groups to multi-centre projects.
Storing samples and data in a Biobank makes it easier for them to be used in any external research project, making it possible to establish collaborations in the projects or signing authorship policies between the people involved.
STAFF
Biobank
Ana María Torres (Scientific Director)
Sonia Camaño (Technical Coordination and Quality Manager)
Bárbara Luna (Laboratory and Training Manager)
Lorien Soto (Laboratory Technical Staff)
Sara Montaña (Laboratory Technical Staff)
BiomodelsBruno Sainz (Coordination of in vivo-PDX models)
Laura Ruiz (Responsible for in vivo-PDX models)
Julie Earl (Coordination of in vitro models-Cell lines and Organoids)
Jorge Villalón (Laboratory Technical Staff)
Javier Rumín (Laboratory Technical Staff)
Main collaboratorsIgnacio Ruiz (Medical Staff in Pathological Anatomy)
Amparo Berlinches (Medical Staff in Pathological Anatomy)
Federico Soria (Head of the Animal Facility and head of the Animal Facility and Experimental Surgery)
María Laura García Bermejo (Head of Biomarkers and therapeuthic targets group)
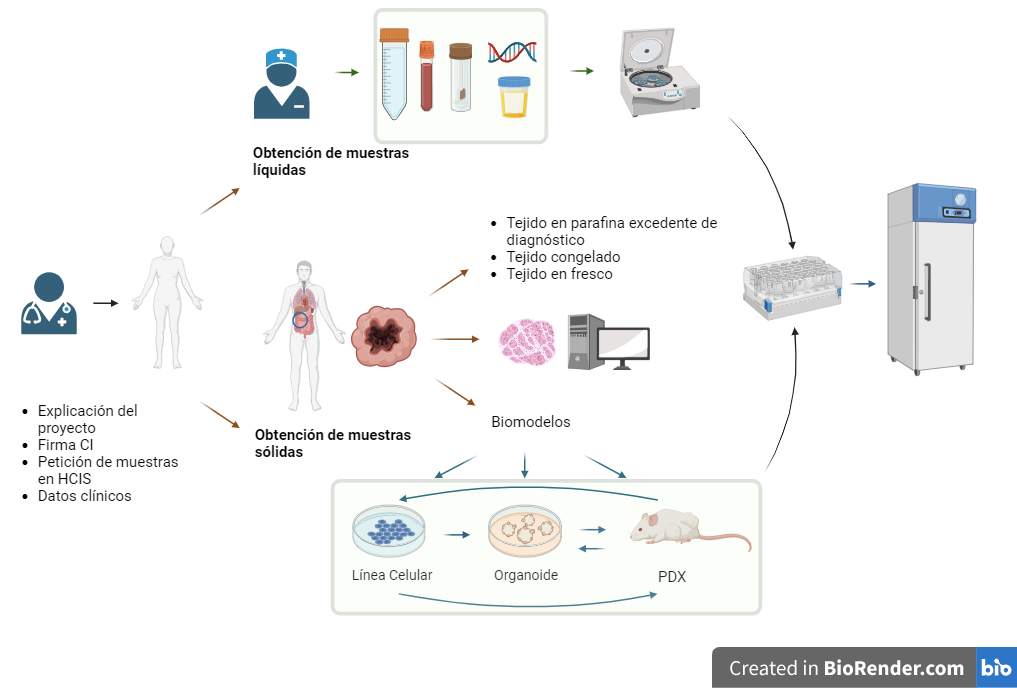
CIRCUIT OF BIOLOGICAL SAMPLES, BIOMODELS AND DATA COLLECTIONS
-
Equipment
- Freezers -80ºC and -20º.
- Temperature control and alarm management systems.
- Nitrogen tanks.
- TRACKER two-dimensional code reader for sample storage tubes.
- Cell culture room.
- Automatic cell counter.
- Refrigerated centrifuges.
- Web application for the registration and exploitation of samples, and for the management of storage space (Bio-e-bank, VITRO).
- MOTIC tissue scanner
-
Liquid and solid samples
Biobank manages, processes and stores liquid and solid samples for use in research.
In the catalogue you can consult the information of the collections related to the associated pathology, number of cases, type of samples, extraction times, processing conditions, inclusion criteria, repository and active/inactive recruitment status.
Collections marked with an (*) have not only the minimum information associated with the donation and the samples, but also additional information in at least 80% of the cases. This information is defined specifically for each of the pathologies in collaboration with the Depositary responsible for the collection of samples. These data can be of a clinical nature, follow-up, anthropometric, biochemical, pharmacological treatment, habits, family history, etc. They can be consulted in the ANNEX DATA PAPERS ASSOCIATED WITH SAMPLES
DOWNLOAD DETAILED CATALOGUE OF SAMPLES
Haematic derivatives
Serum, plasma, blood without plasma, whole blood or cells to viability.
Other liquid samples
Faeces, saliva, hair, urine, bronchoalveolar lavage fluid, among others.
Tissues
The use of tissue will always be surplus to diagnosis, without posing a risk to the clinical diagnosis of patients, and will always be supervised by expert professionals from Anatomical Pathology.
Frozen tissue blocks in OCT, fresh frozen tissue, fresh tissue, PDX tissue frozen to viability and fresh PDX tissue.
Management of paraffin-embedded blocks from the Anatomical Pathology diagnostic surplus archive.
LIQUID AND SOLID SAMPLE COLLECTIONS
I. Renal diseases
- Renal failure in heart surgery with extracorporeal circulation* (43 cases)
- Acute renal failure* (31 cases)
- Donors with expanded criteria and renal transplant recipients
- Renal transplant recipients (27 cases)
- Chronic and acute kidney disease (70 cases)
- Glomerular kidney pathology* (88 cases)
- Predisposition to acute renal failure associated with cardiac surgery* (68 cases)
- HIV-associated acute renal failure (259 cases)
- Membranous glomerulonephritis (23 cases)
II. Oncological diseases
- Tumour bank of tissue samples included in OCT (2607 cases)
- Pre-treatment and pre-surgery cancer (615 cases)
- Familial or hereditary cancer assessment (795 cases and healthy people)*.
- Pre-colonoscopy (early detection of colorectal cancer)* (97 cases)
- Colorectal cancer (22 cases)
- Pituitary tumours (70 cases)
- Pancreatic cancer (familial and sporadic) and high-risk cases* (335 cases) 383
- Haematopoietic progenitor pre-transplantation* (299 cases)
- Breast cancer (104 cases)
- Lung cancer (I)* (12 cases)
- Lung cancer (II)* (116 cases)
- Lung cancer (III)* Tumour nodules (475) Benign nodules (40) and No nodules or control (187)
- Renal cell cancer (16 cases)
- Digestive tumours (25 cases)
- Breast cancer and associated risk factors (8 cases)
- Colon adenocarcinoma (149 cases)
- Gynaecological tumours (6 cases)
- Cancer of unknown origin (1 case)
- Cancer with immunotherapy* (39 cases)
- Microbiota transplantation in advanced lung cancer treated with immunotherapy (23 cases)
- Lung cancer and living relatives* (30 cases and 25 control) Glioblastoma (11 cases)
- Sarcoma (6 cases)
- Thyroid cancer. Treated (27 cases) and Untreated (2 cases)
- Acute Myeloid Leukaemia (2 cases)0
- Stage II-III Rectal Cancer treated with RT (3 cases)
- Advanced Non-Small Cell Lung Cancer treated with immunotherapy (18 cases)
III. Metabolic diseases
- Metabolic bone diseases* (8 cases)
- Diabetes mellitus type 2 (144 cases)
- Obesity (I)* (114 cases)
- Obesity (II)* (840 cases and 20 controls)
- Obesity. Follow-up of bariatric surgery patients (laparoscopic gastric bypass 22 cases and vertical gastrectomy 10 cases).
- Haemochromatosis (2337 cases)
IV. Cardiovascular diseases- Pulmonary thromboembolism (560 cases)
- Coronary artery disease (881 cases)
- Suspected acute myocardial infarction (13 cases)
- Familial heart disease and cardiomyopathy (335 cases)
- Acute myocardial infarction (I) (237 cases)
- Acute coronary syndrome in children under 55 years of age* (53 cases and 41 control)
- Chronic heart failure (100 cases)
- Isolated triscuspid regurgitation (133 cases and 50 control)
- Mastocyte activation in acute coronary syndrome (11 cases)
- Heart failure II (16 cases)
- Heart failure in very elderly patients (112 cases)
V. Liver diseases
- Chronic liver disease (520 cases)
- Vascular liver disease (181 cases)
VI. Neurological diseases- Huntington's disease* (25 cases)
- Stroke* (9 cases)
- Neurodegenerative diseases* (160 cases)
- VII. Dermatological diseases
- Frontal fibrosing alopecia and folliculitis decalvans (413 cases)
- Psoriasis* (120 cases)
- Psoriasis and Mediterranean diet (4 cases and 6 control)
- Alopecia areata total or universal* (75 cases)
- Atopic dermatitis* (53 cases and 24 control)
VIII. Multi-organ failure- Multi-organ failure* (83 cases)
IX. Infectious diseases
- Severe sepsis (53 cases)
- COVID-19* (703 cases)
X. Rare diseases
- Splenomegaly and thrombopenia (9 cases)
- Cystic fibrosis* (41 cases)
- Hereditary haemorrhagic telangiectasia* (100 cases)
- Giant cell arteritis (15 cases and 15 control)
- Hereditary spastic ataxia/paraparesis* (47 cases)
- Fabry disease (16 cases and 16 control)
XI. Systemic and Autoimmune Diseases- Systemic and Autoimmune Diseases (280 cases)
XII. Controls
- Blood bank donors* (284 cases)
- Non-tumour donors* (73 cases)
-
Biomodels
COLLECTIONS WITH BIOMODELS
PDXS of oncological diseases
Of thoracic origin:
- lung adenocarcinoma (6 cases)
- Carcinoma of the lung (4 cases)
- Sarcomas (5 cases)
- Colon metastases (1 case)
- Breast metastasis (2 cases)
Digestive origin:
- Adenocarcinoma of the colon (12 cases)
- Pancreatic adenocarcinoma (11 cases)
- Cholangiocarcinoma of the Pancreas (2 cases)
- Neuroendocrine Tumour of the Pancreas (1 case)
ORGANOIDS of oncological diseases
Of digestive origin:- Adenocarcinoma of the colon (4 cases)
* Consult the number of samples available for each case.
The generation of biomodels from cells extracted from a human organ makes it possible to increase the amount of biological material for use in research.
They are replicas that make it possible to study health solutions with a direct impact on patients and society. They are a key step on the road to personalised medicine.Biobank of Biomodels (in vitro): 2D culture and organoids
In vitro tumour models are essential in translational cancer research. They allow the identification of new therapeutic targets, the study of tumour metastasis, vascularity and the extracellular matrix in the maintenance and progression of tumours.
2D monolayer cultures are adherent cultures of cells in a single layer liquid culture medium. They are easy to grow and maintain and are useful tools for basic studies (genetics, viability, proliferation, vitality, etc.). Commercial cell lines can be used or primary cultures can be established from patient tumour samples.
Organoids are self-organised cultures that require advanced cell culture techniques and culture media with specific growth factor cocktails and a biological basement membrane that mimics the extracellular environment. They are more representative of the original primary tumour and provide a useful preclinical model for the investigation of different tumour types. These cultures provide tools such as drug screening assays.Biobank of Biomodels (in vivo): Patient derived xenograft (PDX)
The use of biomodels is fundamental in the different stages of biomedical research, from the discovery of molecules, genes or metabolic pathways involved in diseases to pre-clinical trials of new drugs.
The Biobank is committed to oncological research through the generation of PDX (Patient Derived Xenograft). These animal models are created by directly implanting a section of tumour tissue from the patient into an immunocompromised mouse. This biomodel is able to recapitulate and maintain the characteristics of the original tumour at the molecular and genetic level, along with the histological architecture. This allows us to intensively study the alterations that cause tumours and to discover possible therapeutic targets against them. In addition, these PDXs retain the patient's pharmacological response, allowing us to test the efficacy of alternative or experimental compounds.
Each patient has his or her own biomodel (reflecting the diversity and heterogeneity of the population) with unique characteristics but with benefits for society as a whole.
-
Services portfolio
Based on the technical and training possibilities of the Biobank staff, other support services can be provided to interested researchers, including:
- Integrated management (shipment of samples nationally and internationally, clinical data management, review of bioethical aspects and advice on handling of human biological samples and Quality Management System).
- Preparation and drafting of the project report for the application procedures for the evaluation of the research project before the relevant bodies, depending on the case (Research Ethics Committee, Qualifying Body, Community of Madrid and Animal Experimentation Ethics Committee).
- Technical support for Clinical Trials
- Technical support for the processing and preservation of samples.
- Generation of patient-derived xenografts (PDX)
- Preclinical trials
-
Rates
SERVICE DESCRIPTION BASE COST RATE BIOBANK 1 Serum Aliquot (250μL-500μl) 9,44 € 2 Aliquot Plasma EDTA (250μL-500μl) 9,41 € 3 Aliquot EDTA Blood without plasma or buffy coat (250μL-500μl) 9,41 € 4 Plasma CITRATE Aliquot (250μL-500μl) 9,42 € 5 PBMCs mononuclear cells to viability 14,39 € 6 Mononuclear PBMCs dry pellet cells 12,77 € 7 Whole blood - Aliquot 3,50 € 8 Stool - Aliquot 6,05 € 9 RNAlater Stool - Aliquot 11,44 € 6 Aliquot Urine (250μL) or Sediment (10ml) 9,59 € 7 OCT frozen cut tissue Management by case 3,36 € 8 Paraffin block tissue Management by case 1,70 € 13 Request for samples cession Junior hour rate 40 € Support technician hour cost 20 € 14 Sample Shipping Management Junior hour rate 40 € Support technician hour cost 20 € BIOMODELS 15 PDX establishment Generation of patient-derived xenograft (PDX) by implantation of a surgically extracted human tumor biopsy in an immunosuppressed mouse. The tumor generated is called px1 and comes directly from the patient's tissue. 472,2 € 16 PDX expansion Generation of patient-derived xenograft (PDX) by implantation of a human-murine tumor biopsy extracted from an immunocompromised mouse. The tumor generated is called the corresponding pass (e.g. px3 - third pass of the tumor from the original biopsy) and comes directly from mouse tissue. 494,7 € For services not listed in the table, a budget adapted to the specific needs of each collection, project or service will be drawn up.

