Burgaz S, Navarro E, Rodríguez-Carreiro S, Navarrete C, Garrido M, Lastres-Becker I, Chocarro J, Lanciego JL, Muñoz E, Fernández-Ruiz J. Investigation in the cannabigerol derivative VCE-003.2 as a disease-modifying agent in a mouse model of experimental synucleinopathy
Behav Brain Funct. 2024
"PPAR-γ activation with cannabinoids may serve as a neuroprotective therapy against α-synucleinopathies as Parkinson's disease". - Dr Sonia Burgaz and Dr Javier Fernández-Ruiz -
Summary:
Background: The cannabigerol derivative VCE-003.2, which has activity at the peroxisome proliferator-activated receptor-γ, has afforded neuroprotection in experimental models of Parkinson's disease (PD) based on mitochondrial dysfunction (6-hydroxydopamine-lesioned mice) and neuroinflammation (LPS-lesioned mice). Now, we aim to explore VCE-003.2 neuroprotective properties in a PD model that also involves protein dysregulation, other key event in PD pathogenesis.
Methods: To this end, an adeno-associated viral vector serotype 9 coding for a mutated form of the α-synuclein gene (AAV9-SynA53T) was unilaterally delivered in the substantia nigra pars compacta (SNpc) of mice. This model leads to motor impairment and progressive loss of tyrosine hydroxylase-labelled neurons in the SNpc.
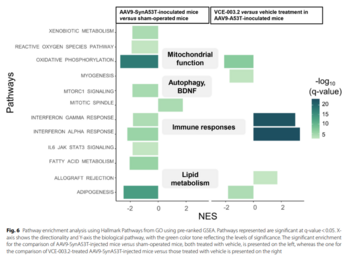
Results: Oral administration of VCE-003.2 at 20 mg/kg for 14 days improved the performance of mice injected with AAV9-SynA53T in various motor tests, correlating with the preservation of tyrosine hydroxylase-labelled neurons in the SNpc. VCE-003.2 also reduced reactive microgliosis and astrogliosis in the SNpc. Furthermore, we conducted a transcriptomic analysis in the striatum of mice injected with AAV9-SynA53T and treated with either VCE-003.2 or vehicle, as well as control animals. This analysis aimed to identify gene families specifically altered by the pathology and/or VCE-003.2 treatment. Our data revealed pathology-induced changes in genes related to mitochondrial function, lysosomal cell pathways, immune responses, and lipid metabolism. In contrast, VCE-003.2 treatment predominantly affected the immune response through interferon signaling.
Conclusion: Our study broadens the neuroprotective potential of VCE-003.2, previously described against mitochondrial dysfunction, oxidative stress, glial reactivity and neuroinflammation in PD. We now demonstrate its efficacy against another key pathogenic event in PD as α-synuclein dysregulation. Furthermore, our investigation sheds light on the molecular mechanisms underlying VCE-003.2 revealing its role in regulating interferon signaling. These findings, together with a favorable ADMET profile, enhance the preclinical interest of VCE-003.2 towards its future clinical development in PD.
Why do you highlight this publication?
This publication closes a series of subsequent studies aimed at investigating the therapeutic value of the cannabigerol derivative VCE-003.2, which have revealed promising neuroprotective properties against key events (e.g., mitochondrial dysfunction, oxidative stress, glial reactivity, neuroinflammation, protein dysregulation) in the pathogenesis of Parkinson's disease. The interest of this last publication is twofold: (i) it is the first demonstration of its efficay in a model of α-synucleinopathy; and (ii) it includes a transcriptomic analysis revealing the major enriched pathways involved in the pathology and the therapeutic effect of VCE-003.2.
Publication commented by:
Dr. Sonia Burgaz García-Oteyza and Dr. Javier Fernández Ruiz
Department of Biochemistry and Molecular Biology,
Faculty of Medicine, Complutense University
NEURODEGENERATIVE DISEASES: THERAPY DEVELOPMENT - IRYCIS