Simón S, den F, García D, Skrempou G, Paraíso J, Aguilera A, Nieto M, Werkman TR, Guzmán M, Chameau P, Galve I.. Prenatal downregulation of CB1 cannabinoid receptors in the mouse prefrontal cortex disrupts cortical lamination and induces a transcriptional signature associated with social interaction deficits
J Neurosci. 2025
"The CB1 cannabinoid receptor regulates projection neuron development of the prefrontal cortex and social behaviour". - Dr. Ismael Galve-Roperh
Summary:
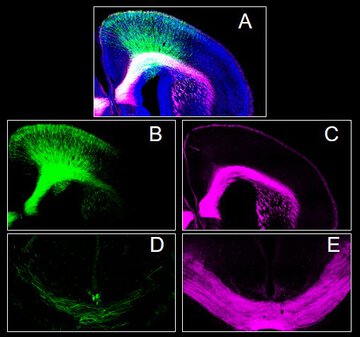
CB1 cannabinoid receptors (CB1Rs) are the proteins that mediate the actions of both endogenous brain molecules known as endocannabinoids and plant-derived compounds from cannabis, including THC. During prenatal development, CB1Rs regulate key processes such as neuronal differentiation, migration, and axonal guidance. In this study, we investigated the long-term consequences of transient prenatal CB1R absence on the development of the mouse prefrontal cortex. Embryonic loss of CB1Rs caused a disruption in neuronal migration, preventing neurons from reaching their final destinations. As a result, neurons misplaced in inappropriate cortical layers displayed altered activity and axonal projection properties compared to neighboring native neurons. Moreover, the absence of CB1Rs during neuronal development produced a distinct molecular gene expression profile that partially overlapped with susceptibility genes for intellectual disability and autism. Together, these neurodevelopmental alterations led to lasting impairments in adult social interaction and motor behavior. These findings emphasize the critical role of CB1Rs in shaping the development of prefrontal cortex projection neurons and provide insight into how altered endocannabinoid signaling may contribute to vulnerability to neuropsychiatric disorders.
Why do you highlight this publication?
This publication uncovers novel insights into the role of the endogenous cannabinoid system in prenatal prefrontal cortex development. We show that the loss of CB1R signaling leads to neuronal migration arrest, disrupted contralateral brain connectivity, and a gene expression profile that shows similarity with neuropsychiatric disorders such as intellectual disability, and autism. These developmental disruptions culminate in long-lasting deficits in social interaction in adult mice. Beyond its mechanistic findings, the study has important implications for understanding the impact of prenatal cannabinoid exposure on brain development and for identifying pathways that underlie vulnerability to psychiatric conditions.
Publication commented by:
Dr. Ismael Galve-Roperh
Department of Molecular Biochemistry and Biology. UCM
NEURODEGENERATIVE DISEASES: PATHOGENIC MECHANISMS group. IRYCIS